Nanosized diagnostic tools for personalized medicine . “In vivo” and “in vitro” applications.
Actively and passively targeted nanoparticles (liposomes, PLGA etc) are proposed for the delivery of magnetic resonance imaging (MRI) contrast agents for the detection of malignancies and for monitoring the effects of therapeutic agents.

Ferritin Decorated PLGA/Paclitaxel Loaded Nanoparticles Endowed with an Enhanced Toxicity Toward MCF‑7 Breast Tumor Cells.
Polylactic and glycolic acid nanoparticles (PLGA-NPs), coated
with L-ferritin, are exploited for the simultaneous delivery of
paclitaxel and an amphiphilic Gd based MRI contrast agent into
breast cancer cells (MCF7). L-ferritin has been covalently
conjugated to the external surface of PLGA-NPs exploiting NHS
activated carboxylic groups. 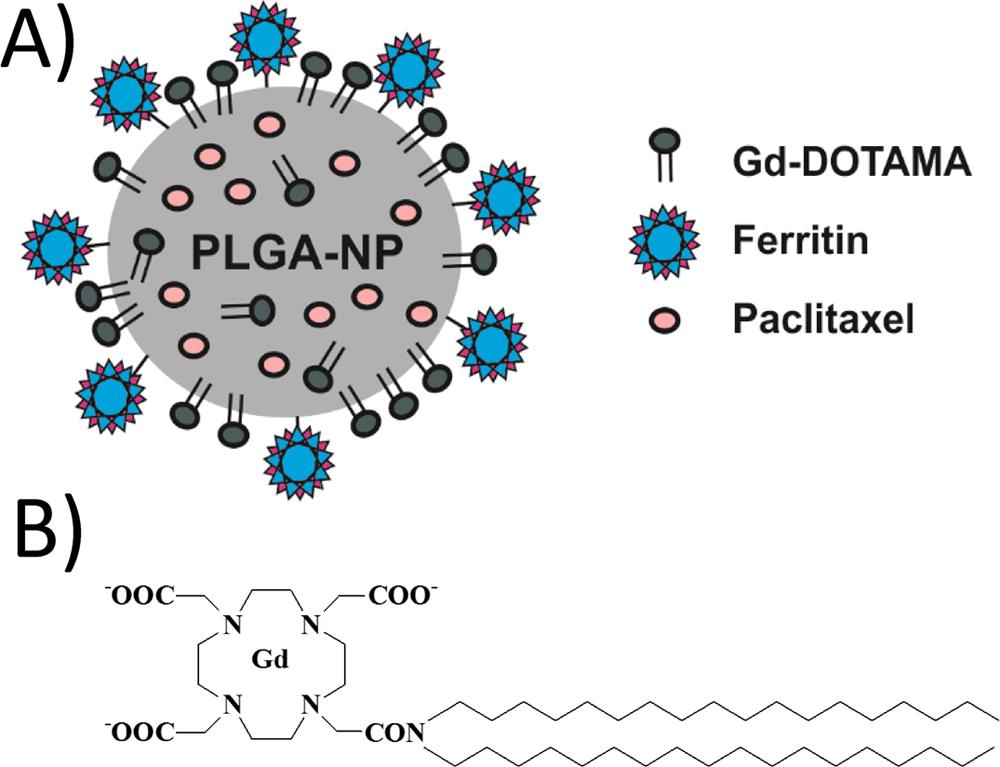 The results confirmed that nanoparticles decorated with
L-ferritin have many advantages with respect to both
albumin-decorated and nondecorated particles. Ferritin moieties
endow PLGA-NPs with targeting capability, exploiting SCARA5
receptors overexpressed by these tumor cells, that results in
an increased paclitaxel cytotoxicity. Moreover, protein coating
increased nanoparticle stability, thus reducing the fast and
aspecific drug release before reaching the target. The
theranostic potential of the nanoparticles has been
demonstrated by evaluating the signal intensity enhancement on
T1-weighted MRI images of labeled MCF7 cells. The results were
compared with that obtained with MDA cells used as negative
control due to their lower SCARA5 expression.
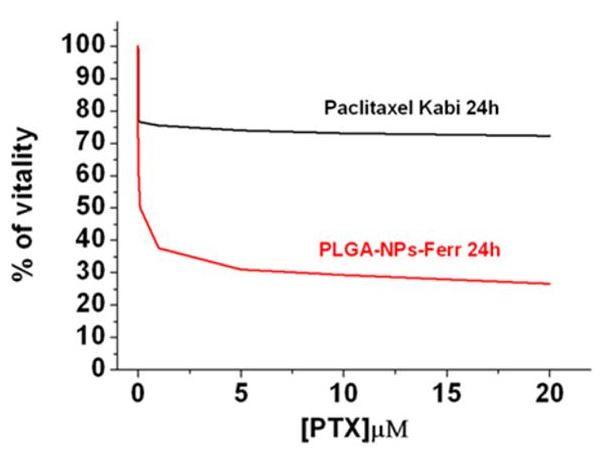
The results confirmed that nanoparticles decorated with
L-ferritin have many advantages with respect to both
albumin-decorated and nondecorated particles. Ferritin moieties
endow PLGA-NPs with targeting capability, exploiting SCARA5
receptors overexpressed by these tumor cells, that results in
an increased paclitaxel cytotoxicity. Moreover, protein coating
increased nanoparticle stability, thus reducing the fast and
aspecific drug release before reaching the target. The
theranostic potential of the nanoparticles has been
demonstrated by evaluating the signal intensity enhancement on
T1-weighted MRI images of labeled MCF7 cells. The results were
compared with that obtained with MDA cells used as negative
control due to their lower SCARA5 expression.
 L.N. Turino et al,
Bioconjug Chem. 2017 Apr 19;28(4):1283-1290
L.N. Turino et al,
Bioconjug Chem. 2017 Apr 19;28(4):1283-1290
A Quantitative Relaxometric Version of the ELISA Test for the Measurement of Cell Surface Biomarkers
Quantitative measurement of marker expression in diseased cells
is still a topic of considerable interest and different
methodologies are currently under intense scrutiny. This work
aims at developing an in vitro diagnostic method based on the
release of paramagnetic species from relaxometrically “silent”
liposomes operated by the action of a phospholipase A2 (PLA2)
previously targeted to the epitope of interest. The released
paramagnetic species causes an increase of the longitudinal
water proton relaxation rate proportional to the number of PLA2
bound to the cell outer surface. 
The sensitivity of the herein proposed method, named R-ELISA, was attempted in the detection of folate receptor expression on human ovarian cancer cells by functionalizing PLA2 with folic acid. Receptor/cell number of 8.3 105 has been measured on IGROV-1 cells. The R-ELISA assay can detect nanomolar cell suspension receptor concentrations and has been validated by well-established spectrofluorimetric procedures.
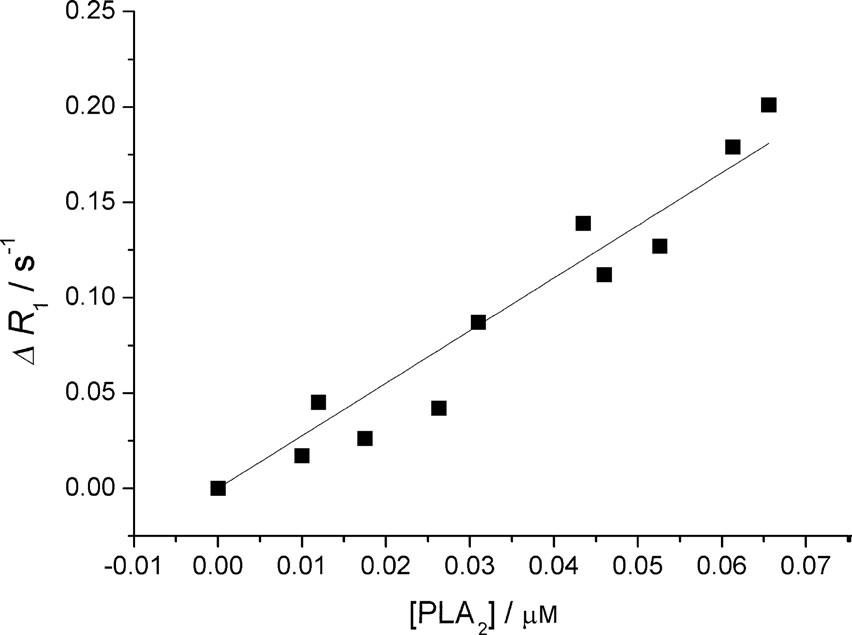
Figure: Calibration curve obtained by measuring DR1 versus [PLA2].
D. Alberti et al Angew. Chem. Int. Ed. 2014, 53, 3488 –3491Combined Delivery and Magnetic Resonance Imaging of Neural Cell Adhesion Molecule–Targeted Doxorubicin- Containing Liposomes in Experimentally Induced Kaposi's Sarcoma
The aim of the present study was to investigate whether neural cell adhesion molecule (NCAM)–targeted liposomes may enhance drug delivery and allow MRI in a severe combined immunodeficient mouse model of NCAM-positive Kaposi's sarcoma. NCAM-binding peptide–coated liposomes loaded with both doxorubicin and a lipophilic gadolinium (Gd) derivative were generated. NCAM-targeted liposomes induced an enhanced in vitro doxorubicin internalization within Kaposi's cells as detected by MRI with respect to untargeted polyethylene glycol liposomes. Internalization resulted in enhanced apoptosis. In vivo weekly administration of NCAM-targeted liposomes induced a significant reduction of tumor mass and vascularization and enhanced cell necrosis and apoptosis with respect to untargeted liposomes. These effects were associated with an enhanced concentration of doxorubicin within the tumor and a reduced systemic toxicity of doxorubicin. By electron microscopy, NCAM-targeted liposomes were detected mainly within tumor cells whereas the untargeted liposomes were mainly accumulated in the extracellular space.
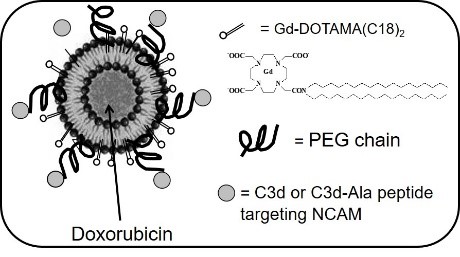
In conclusion, targeting NCAM may be a suitable strategy for specific drug delivery and imaging by liposomes in NCAM-expressing tumors.